The Baghdad battery or the Parthian battery is a set of three artefacts which was found together: a ceramic pot, a tube of copper, and a rod of iron. It was discovered in modern khujut, Rabu, Iraq close to the metropolis of Ctesiphon, the capital of the Parthian.
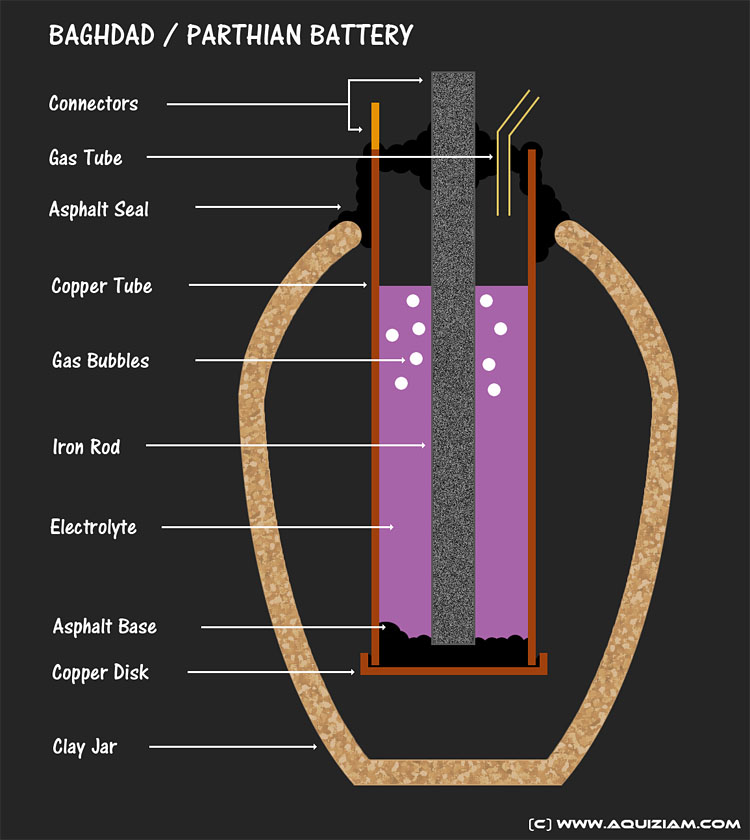
The Baghdad battery, otherwise known as the Parthian battery, was an artefact hypothesized to be an ancient version of a battery. Found in 1938 by a German archaeologist, the Baghdad Battery' could be 2,000 years old and consists of a clay jar, a copper cylinder, and an iron rod.
The inner working of the Baghdad battery
Fill the jar with an acidic liquid, such as vinegar or fermented grape juice, and you have yourself a battery capable of generating a small current. The acidic liquid permits the flow of electrons from the copper tube to the iron rod when two metal terminals are connected.


What is the Baghdad battery actually used for?
Scientists believe that the Baghdad battery (If that is their correct function) was used to electroplate items such as putting a layer of one metal (Gold) into the surface of another (Sliver), a method still practised in Iraq today.
This battery produces about 1.5 to 2 volts. Using the lower voltage 1.5v which is the same as a common AA battery. if you take 18 Baghdad jars and connect them in series you will produce 18 volts.

How does grape juice form electrons?
The Movement is rapid at high temperature and it causes the electrons to be knocked off from the molecules, forming the electron-ion cloud. As the temperature increases plasma is formed.
- Beside Grape juice is high in electrolytes
What is Plasma
Plasma is a hot ionized gas consisting of equal numbers of positively charged ions and negatively charged electrons.
The Baghdad battery, otherwise known as the Parthian battery, was an artefact hypothesized to be an ancient version of a battery. Found in 1938 by a German archaeologist, the Baghdad Battery' could be 2,000 years old and consists of a clay jar, a copper cylinder, and an iron rod.
The inner working of the Baghdad battery
Fill the jar with an acidic liquid, such as vinegar or fermented grape juice, and you have yourself a battery capable of generating a small current. The acidic liquid permits the flow of electrons from the copper tube to the iron rod when two metal terminals are connected.


What is the Baghdad battery actually used for?
Scientists believe that the Baghdad battery (If that is their correct function) was used to electroplate items such as putting a layer of one metal (Gold) into the surface of another (Sliver), a method still practised in Iraq today.
This battery produces about 1.5 to 2 volts. Using the lower voltage 1.5v which is the same as a common AA battery. if you take 18 Baghdad jars and connect them in series you will produce 18 volts.


How does grape juice form electrons?
The Movement is rapid at high temperature and it causes the electrons to be knocked off from the molecules, forming the electron-ion cloud. As the temperature increases plasma is formed.
- Beside Grape juice is high in electrolytes
What is Plasma
Plasma is a hot ionized gas consisting of equal numbers of positively charged ions and negatively charged electrons.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.